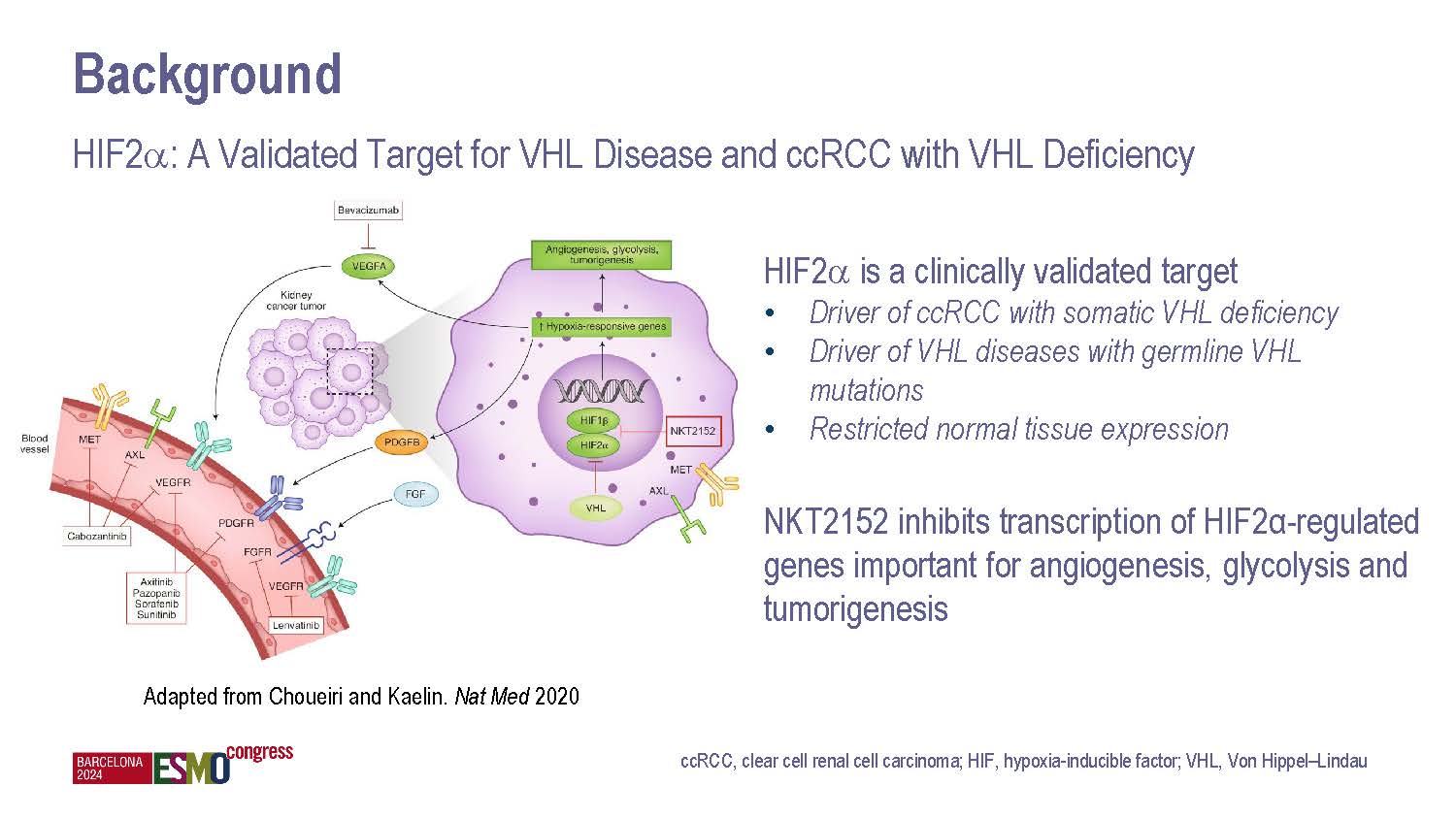
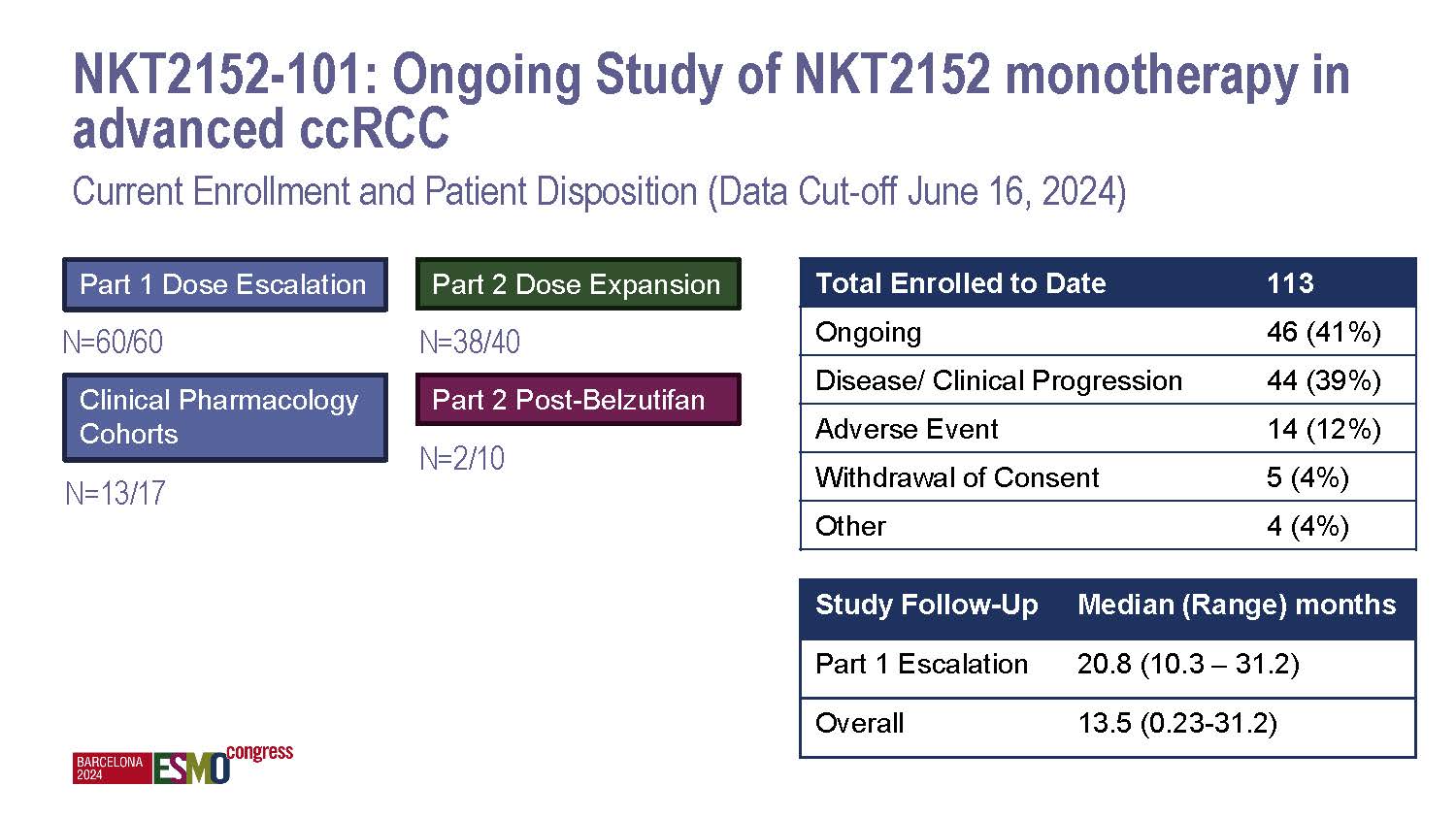
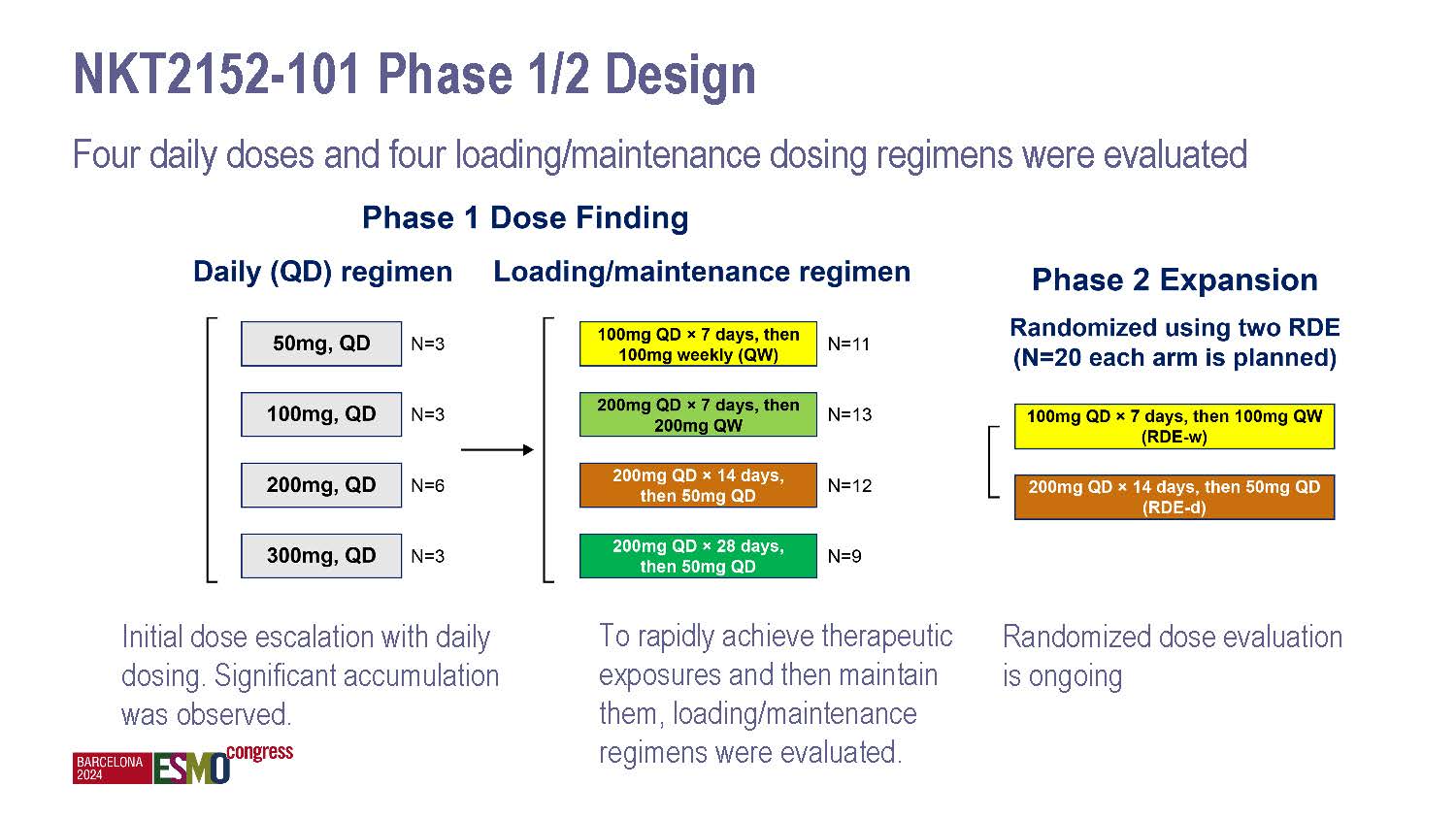
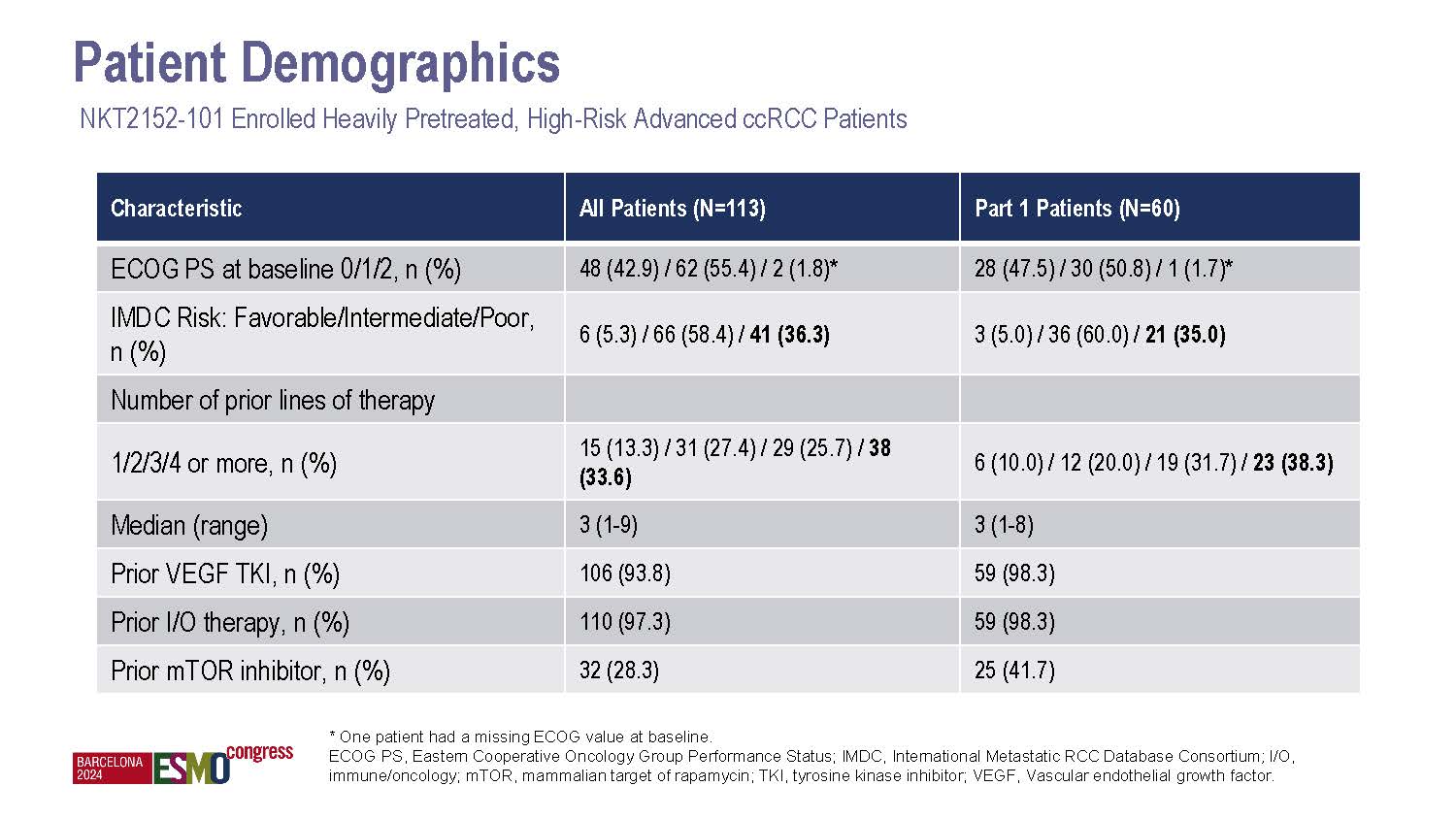
NiKang Therapeutics Appoints Anne E. Borgman, M.D., to Board of Directors
See the press release on Business Wire
See the press release on Business Wire
See the press release on Business Wire
See the press release on EIN Newswire
See the press release on EIN Newswire